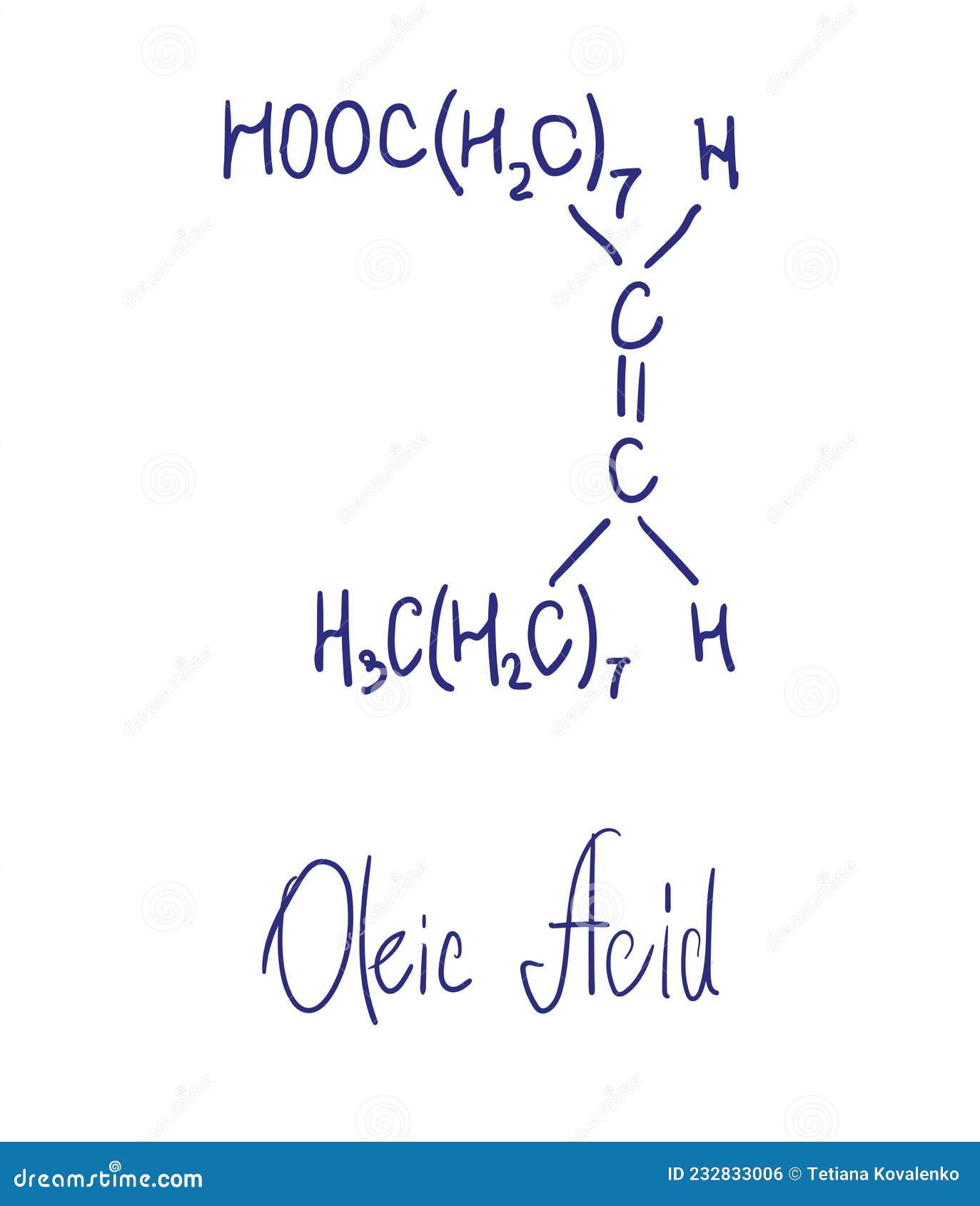
To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both.
To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oct 18, 2011 · find the precision your measurements of the length and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. Oct 12, 2016 · we use the avogadro’s number to determine the molecules per mole of the fatty substance called oleic acid. Oleic acid is an organic acid, or fatty acid and oleic acid is in the. So the calculated height is more accurately thought of as the length of an oleic acid molecule. What is meant by a monolayer? Why is it necessary to dilute the oleic acid for. Nov 4, 2007 · find the precision your measurements of the length, width and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. • make sure the tray used is large (at least 30 × 40 cm).
To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oct 18, 2011 · find the precision your measurements of the length and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. Oct 12, 2016 · we use the avogadro’s number to determine the molecules per mole of the fatty substance called oleic acid. Oleic acid is an organic acid, or fatty acid and oleic acid is in the. So the calculated height is more accurately thought of as the length of an oleic acid molecule. What is meant by a monolayer? Why is it necessary to dilute the oleic acid for. Nov 4, 2007 · find the precision your measurements of the length, width and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. • make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length. By using a method similar to his, it is possible to determine the approximate length of an oleic acid molecule. Learn how to measure the size of oleic acid. Learn the thin layer measurement test and how it is used for oleic acid. Explore more related concepts at byju's.
To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oct 18, 2011 · find the precision your measurements of the length and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. Oct 12, 2016 · we use the avogadro’s number to determine the molecules per mole of the fatty substance called oleic acid. Oleic acid is an organic acid, or fatty acid and oleic acid is in the. So the calculated height is more accurately thought of as the length of an oleic acid molecule. What is meant by a monolayer? Why is it necessary to dilute the oleic acid for. Nov 4, 2007 · find the precision your measurements of the length, width and mass of oleic acid molecules. Express the precision as a percent error by dividing the ± amount by the size of the. • make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length. By using a method similar to his, it is possible to determine the approximate length of an oleic acid molecule. Learn how to measure the size of oleic acid. Learn the thin layer measurement test and how it is used for oleic acid. Explore more related concepts at byju's.
How To Identify And Avoid Unsafe Drivers
Discover The Vivica Clinic Advantage In Windham, CT
The Cotton Gin And The Concert Hall: Eli Whitney's Dual Life