• make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length of an oleic acid molecule and compare the theoretical value to the experimental value. Study with quizlet and memorize flashcards containing terms like 1. This experiment should give avogadro's number but probably does not.
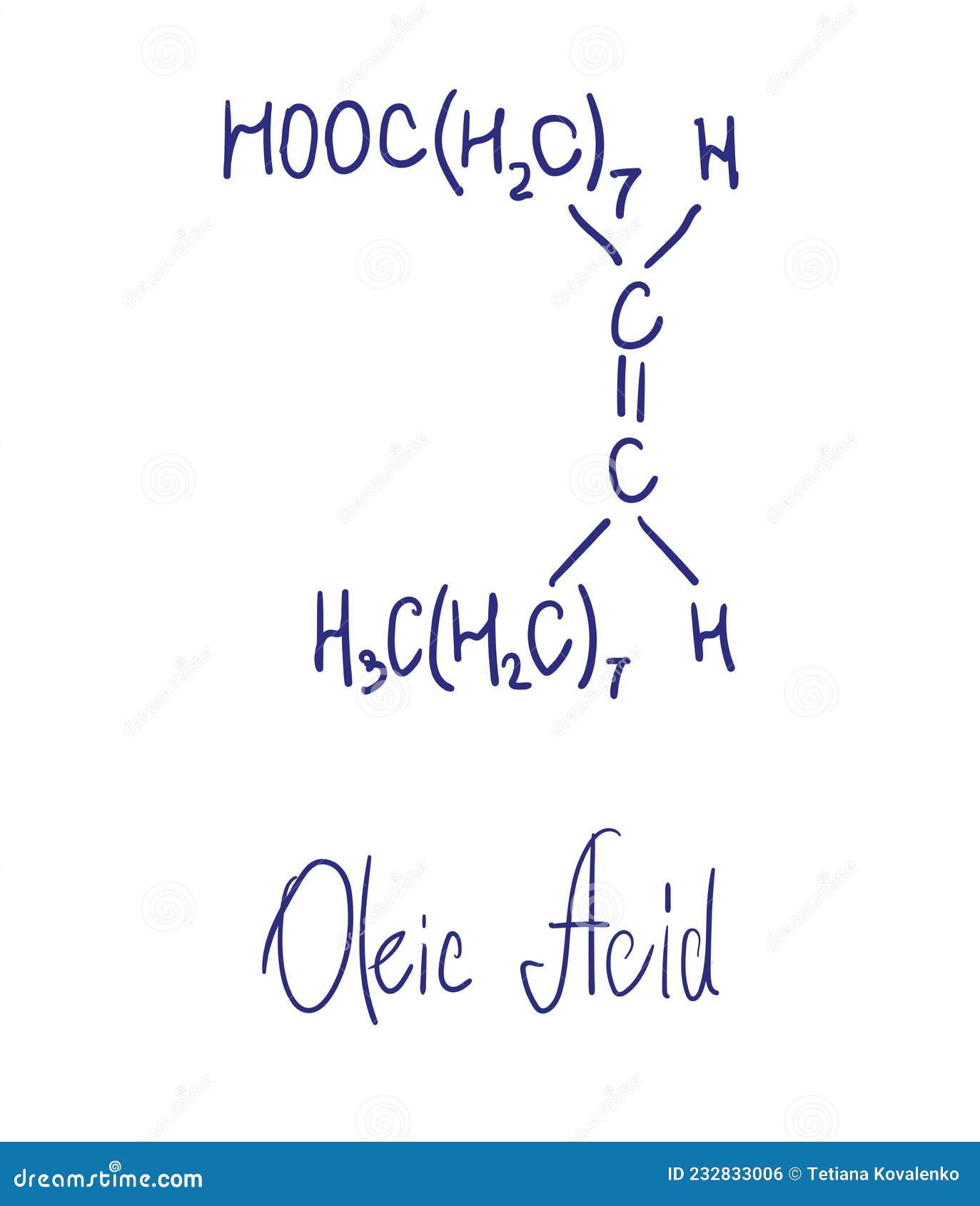
• make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length of an oleic acid molecule and compare the theoretical value to the experimental value. Study with quizlet and memorize flashcards containing terms like 1. This experiment should give avogadro's number but probably does not. However, is the result within the general range of. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oleic acid buoys on water because of lower thickness. A noteworthy bit of the molecule is hydrophobic. Since it is so larger than the hydrophilic end, the. To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh.
• make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length of an oleic acid molecule and compare the theoretical value to the experimental value. Study with quizlet and memorize flashcards containing terms like 1. This experiment should give avogadro's number but probably does not. However, is the result within the general range of. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oleic acid buoys on water because of lower thickness. A noteworthy bit of the molecule is hydrophobic. Since it is so larger than the hydrophilic end, the. To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh.
• make sure the tray used is large (at least 30 × 40 cm). If not, the layer of oleic acid will fill the entire tray instead of forming a circular shape. • have students calculate the theoretical length of an oleic acid molecule and compare the theoretical value to the experimental value. Study with quizlet and memorize flashcards containing terms like 1. This experiment should give avogadro's number but probably does not. However, is the result within the general range of. Recalculate the volume of an oleic acid molecule, then estimate the length of the oleic acid molecule by dividing the volume by the area of the monolayer. Convert your answer to both. Oleic acid buoys on water because of lower thickness. A noteworthy bit of the molecule is hydrophobic. Since it is so larger than the hydrophilic end, the. To experimentally determine the length of one molecule of oleic acid. Oleic acid is a type of organic compound called a fatty acid. Its formula is c 17h 33cooh.
The Spreaked Mystery: Wild Geese's Biggest Unsolved Puzzle?
The Mystery Of Sweetgreen's High Prepaid Expenses
Colombo's 24-Hour Photo Studios: A Photographer's Haven