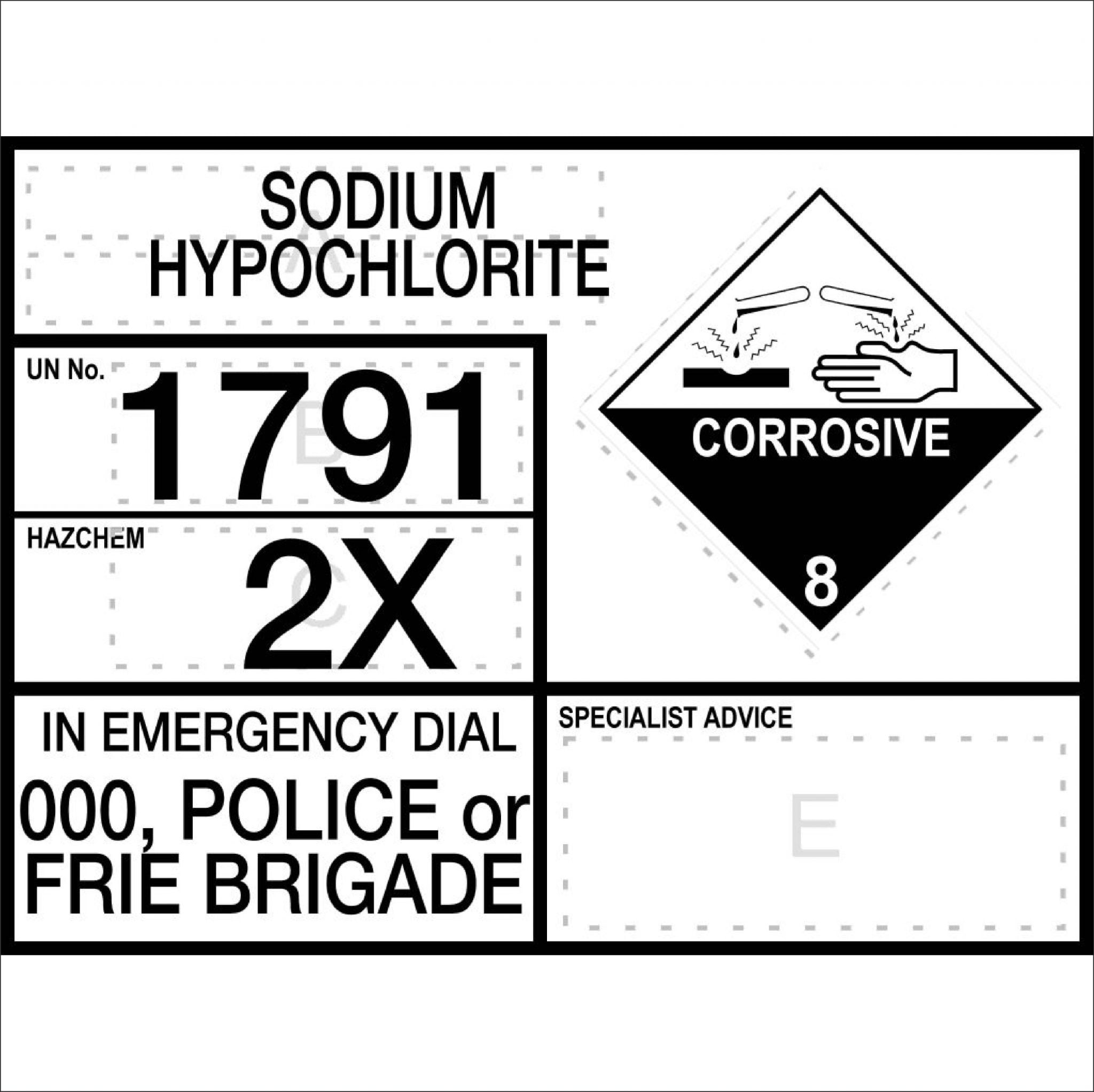
The first stage utilizes sodium hypochlorite (or chlorine gas) to convert cyanide to cyanate at elevated ph (10+). The cyanide and chlorine react to form cyanogen chloride, which is then mixed with more chlorine to form cyanate. The predominant mode of cyanide wastewater treatment is alkaline chlorination. This treatment proceeds in two steps. First, cyanide is oxidized to cyanate by sodium hypochlorite.
The first stage utilizes sodium hypochlorite (or chlorine gas) to convert cyanide to cyanate at elevated ph (10+). The cyanide and chlorine react to form cyanogen chloride, which is then mixed with more chlorine to form cyanate. The predominant mode of cyanide wastewater treatment is alkaline chlorination. This treatment proceeds in two steps. First, cyanide is oxidized to cyanate by sodium hypochlorite. Current industry techniques for management of cyanide ensures the protection of a high ph using sodium hypochlorite reagent. May 15, 1985 · cyanogen bromide in water and seven organic solvents and sodium cyanide in water may safely and efficiently (greater than 99. 7%) be destroyed using sodium hydroxide (1. Dec 16, 2021 · all cyanide waste, solid or liquid, must be made safe by addition to a solution of sodium hypochlorite. Sodium hypochlorite solution (typically 8% available chlorine) is available.
The first stage utilizes sodium hypochlorite (or chlorine gas) to convert cyanide to cyanate at elevated ph (10+). The cyanide and chlorine react to form cyanogen chloride, which is then mixed with more chlorine to form cyanate. The predominant mode of cyanide wastewater treatment is alkaline chlorination. This treatment proceeds in two steps. First, cyanide is oxidized to cyanate by sodium hypochlorite. Current industry techniques for management of cyanide ensures the protection of a high ph using sodium hypochlorite reagent. May 15, 1985 · cyanogen bromide in water and seven organic solvents and sodium cyanide in water may safely and efficiently (greater than 99. 7%) be destroyed using sodium hydroxide (1. Dec 16, 2021 · all cyanide waste, solid or liquid, must be made safe by addition to a solution of sodium hypochlorite. Sodium hypochlorite solution (typically 8% available chlorine) is available.
The first stage utilizes sodium hypochlorite (or chlorine gas) to convert cyanide to cyanate at elevated ph (10+). The cyanide and chlorine react to form cyanogen chloride, which is then mixed with more chlorine to form cyanate. The predominant mode of cyanide wastewater treatment is alkaline chlorination. This treatment proceeds in two steps. First, cyanide is oxidized to cyanate by sodium hypochlorite. Current industry techniques for management of cyanide ensures the protection of a high ph using sodium hypochlorite reagent. May 15, 1985 · cyanogen bromide in water and seven organic solvents and sodium cyanide in water may safely and efficiently (greater than 99. 7%) be destroyed using sodium hydroxide (1. Dec 16, 2021 · all cyanide waste, solid or liquid, must be made safe by addition to a solution of sodium hypochlorite. Sodium hypochlorite solution (typically 8% available chlorine) is available.
5770/8371: The Percentage That's Causing A Stir!
Is Tuy Hoa, Vietnam The Perfect Place For You?
October 4th, 2024: The Day No One Expected